Epidural spinal cord stimulation (SCS) has emerged as a successful treatment for chronic pain disorders such as failed back syndrome, complex regional pain syndrome (CRPS) and peripheral vascular disease.
Cervical SCS is less commonly utilized but also has proven effective for pain syndromes such as upper extremity CRPS, intractable facial pain, angina pectoris and post-amputation limb pain.
While thoracolumbar epidural electrodes are commonly placed with local anesthesia and conscious sedation so the patient may communicate with the surgical team and confirm that regions of pain are satisfactorily treated, placement of surgical cervical epidural leads requires general anesthesia for reasons of safety, patient comfort, and the need for head immobilization.
Although modern multi-contact electrodes have expanded our capability for manipulating the distribution of stimulation current postoperatively, the initial location of the electrode lead is the predominant factor which determines whether stimulation will be effective for treatment of painful regions. Moreover, stimulation of regions not involved in the pain syndrome may cause patient discomfort and therefore limit the therapeutic efficacy of the ultimate treatment by altering the tolerance threshold of stimulation parameters.
Since paddle type electrodes are placed in the epidural space via a partial laminectomy or hemilaminectomy, it can be difficult to control their medial-lateral trajectory. Intra-operative fluoroscopy is helpful for estimating lead laterality but will not ultimately confirm if one or both sides of the spinal cord will receive clinically significant stimulation. Consequently, after the surgeon’s best efforts to position a paddle electrode, there has been a need to develop a methodology to objectively confirm the location of cervical epidural electrodes without patient cooperation.
Somatosensory evoked potentials (SSEP’s) have become a mainstay of neurophysiologic monitoring in spine surgery due to their high sensitivity and specificity for identifying spinal cord injury and proven ability to reduce new postoperative neurological deficits. The potential of SSEPs to assist with localization in the nervous system has been shown by their ability to identify functional regions of the human cortex during brain surgery. Several studies have demonstrated that SCS reduces the amplitudes of short and mid-latency SSEPS, and this decrease of primary somatosensory cortical activity may contribute to the analgesic effect of SCS. We have taken further advantage of our routine SSEP monitoring to correctly lateralize and optimize electrode position during cervical and cervicomedullary SCS surgery.
Data from 44 patients undergoing SSEP monitoring during cervical laminectomy or hemilaminectomy for placement or replacement of dorsal column stimulators were reviewed. Leads were positioned laterally or just off midline and parallel to the axis of the cervical spinal cord to effectively treat what was most commonly a predominant unilateral pain syndrome. During SSEP recording, the spinal cord stimulator lead was activated and intensity increased in increments of 1.0 V to a maximum of 6.0 V. A unilateral reduction or abolishment of SSEP amplitude was regarded as an indicator of lateralized placement of the stimulator. A bilateral diminutive effect on SSEPs was interpreted as a midline or near midline lead placement. Epidural stimulation abolished or significantly reduced SSEP amplitudes in all patients undergoing placement for a unilateral pain syndrome. In 15 patients, electrodes were repositioned intraoperatively to achieve the most robust SSEP amplitude suppression using the lowest epidural stimulation intensity. In all cases where a significant unilateral reduction in SSEP was observed, patient reported postoperative sensory alterations in target locations predicted by intraoperative SSEP changes. Placement of cervical spinal cord stimulators for bilateral pain syndromes often resulted in bilateral, although asymmetric SSEP changes. In no cases were significant changes, other than those induced using the device to directly stimulate the dorsal surface of the spinal cord, observed. No case of new postoperative neurological deficit was observed.
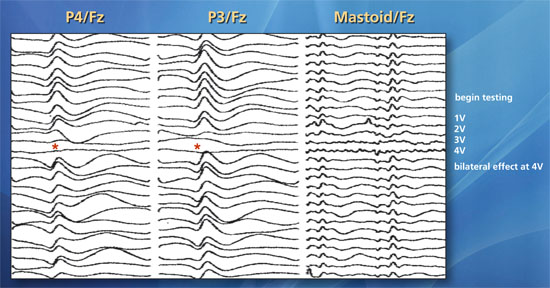
Representative case. A 58-year-old woman who presented with bilateral jaw, shoulder and arm pain underwent placement of a cervical stimulator with desired midline placement. Upper extremity median nerve SSEPs generated by stimulation of the right and left median nerves demonstrated significant bilateral amplitude increases (red asterisks) in response to SCS at 2-4 V providing electrophysiological confirmation of sufficient (midline) electrode placement.
This technique, which utilizes well-established routine SSEP recording, does not require additional training nor does it add significant time to the procedure. We are aware of continued advancements in SCS lead technology and that our efforts in lateralization could potentially be obviated by placing multiple parallel leads or wider paddle leads with multiple rows of embedded electrode contacts. However, in the cervical spine, where the potential for post-laminectomy instability is greater than in the thoracic spine, we feel that a minimal exposure with small laminotomy and limited ligamentous disruption is prudent. Added cost and risk of infection aside, placing more hardware into the cervical epidural space to treat predominantly unilateral pain syndromes arguably exposes the patient to added neurologic risk. SSEPs can safely, easily, and successfully predict the lateralization of an epidural electrode and corresponding stimulation-induced paresthesias in patients undergoing cervical or cervicomedullary spinal cord stimulation. Continuous SSEP monitoring can be used to provide neurophysiological surveillance of final electrode position by alerting the surgical team to an unintended movement of the electrode during anchoring and closing steps.
